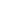Atoms are made of smaller particles(微粒), called electrons(电子), protons(质子), and neutrons(中子). An atom consists of a cloud of electrons surrounding a small, dense nucleus of protons and neutrons. Electrons and protons have a property called electric charge(电荷), which affects the way they interact with each other and with other electrically charged particles. Electrons carry a negative electric charge, while protons have a positive electric charge. The negative charge is the opposite of the positive charge, and, like the opposite poles of a magnet, these opposite electric charges attract one another. Conversely, like charges (negative and negative, or positive and positive) repel one another(互相排斥). The attraction between an atom’s electrons and its protons holds the atom together. Normally, an atom is electrically neutral, which means that the negative charge of its electrons is exactly equaled by the positive charge of its protons.
The nucleus contains nearly all of the mass of the atom, but it occupies only a tiny fraction of the space inside the atom. The diameter of a typical nucleus is only about 1 × 10-14 m (4 × 10-13 in), or about 1/100,000 of the diameter of the entire atom. The electron cloud makes up the rest of the atom’s overall size. If an atom were magnified until it was as large as a football stadium, the nucleus would be about the size of a grape.